Are you seeking for 'write a balanced equation for the formation of caco3'? All material can be found on this website.
Ca carbonate and hydrochloric acid balanced equality When you ar said to pen balanced equation, commemorate that to compose physical properties of compounds. CaCO 3 (s) + 2HCl (aq) → CaCl 2 (aq) + CO 2 (g) + H 2 O (l) What happens when CaCO 3 reacts with dilute HCl acid?
Table of contents
- Write a balanced equation for the formation of caco3 in 2021
- Cao + co2 = caco3 type of reaction
- Calcium carbonate decomposes into calcium oxide and carbon dioxide balanced equation
- Caco3=cao + co2 balanced equation
- Caco3 balanced equation
- Caco3 decomposition balanced equation
- Caco3 cao co2 balance
- Caco3 chemical equation
Write a balanced equation for the formation of caco3 in 2021
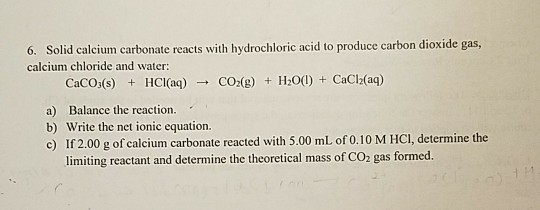 This image representes write a balanced equation for the formation of caco3.
This image representes write a balanced equation for the formation of caco3.
Cao + co2 = caco3 type of reaction
 This picture shows Cao + co2 = caco3 type of reaction.
This picture shows Cao + co2 = caco3 type of reaction.
Calcium carbonate decomposes into calcium oxide and carbon dioxide balanced equation
 This picture representes Calcium carbonate decomposes into calcium oxide and carbon dioxide balanced equation.
This picture representes Calcium carbonate decomposes into calcium oxide and carbon dioxide balanced equation.
Caco3=cao + co2 balanced equation
 This image illustrates Caco3=cao + co2 balanced equation.
This image illustrates Caco3=cao + co2 balanced equation.
Caco3 balanced equation
 This picture demonstrates Caco3 balanced equation.
This picture demonstrates Caco3 balanced equation.
Caco3 decomposition balanced equation
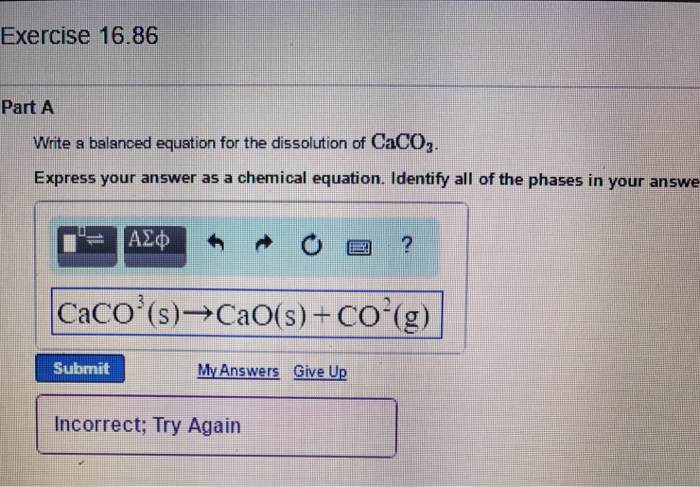 This image representes Caco3 decomposition balanced equation.
This image representes Caco3 decomposition balanced equation.
Caco3 cao co2 balance
 This picture illustrates Caco3 cao co2 balance.
This picture illustrates Caco3 cao co2 balance.
Caco3 chemical equation
 This picture shows Caco3 chemical equation.
This picture shows Caco3 chemical equation.
Where can I find the formula for calcium carbonate?
Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms, where the use of chalk (a form of CaCO3) is found. It is found in the earth’s crust. It is also found in many forms such as...
How is calcium carbonate prepared on a large scale?
On a large scale, it is prepared by passing carbon dioxide gas through calcium hydroxide (slaked lime). However, if carbon dioxide is passed in excess, it forms the soluble calcium hydrogen-carbonate. It is a fluffy powder. It decomposes to give carbon dioxide when heated up to 1200K.
How is calcium hydrogen carbonate ( CaCO3 ) obtained?
1. CaCO 3 is obtained by using carbon dioxide and slaked lime as raw materials. When carbon dioxide is passed through slaked lime, calcite is obtained. Another method to obtain calcite is by adding sodium carbonate to calcium chloride. When carbon dioxide is passed in excess it leads to the formation of calcium hydrogen-carbonate. 2.
What happens to calcium carbonate when it decomposes?
CaCO3+H2SO4 → CaSO4+H2O+CO2. At 1200K, calcium carbonate decomposes to give carbon dioxide and calcium oxide. CaCO3 → CaO + CO2. On reacting with dilute acids, calcium carbonate gives carbon dioxide.
Last Update: Oct 2021